what reagents convert an alcohol to alkyl chloride
Making Alkyl Halides From Alcohols
In today's post we show that treating alcohols with HCl, HBr, or Hi (which all fall nether the catch-all term "HX" where 10 is a halide) results in the formation of alkyl halides. Summary:
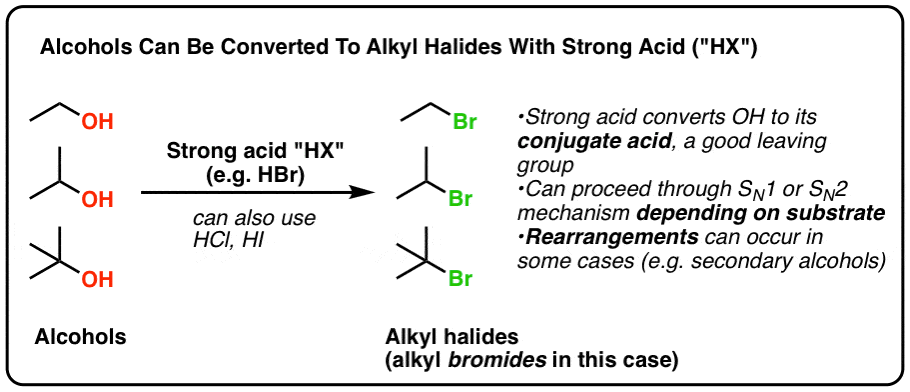
Table of Contents
- Adding Acid To Alcohol Produces A Good Leaving Grouping (H2o)
- Alkyl Halides From Methyl and Master Alcohols via the SN2 Reaction
- Alkyl Halides From Tertiary Alcohols Proceeds Through The SN1 Pathway
- A Good Dominion Of Thumb For Secondary Alcohols With HX: Assume SN1
- Rearrangements In The Formation of Alkyl Halides From Alcohols
- Rearrangement Example #ane: Hydride Shift
- Rearrangement Example #2: Alkyl Shift
- Rearrangement Example #iii: A Special Case of Alkyl Shift – Ring Expansion and Contraction
- What Doesn't Work?
- Summary: Alkyl Halides From Alcohols
- (Advanced) References and Farther Reading
ane. Adding Acid To Alcohol Results In A Good Leaving Grouping
We've said many times in this series of posts that alcohols are poor substrates for SDue north1 and SNii reactions. That'south considering the hydroxyl ion (HO-) is a poor leaving group, and therefore not probable to either 1) depart of its ain accord, leaving behind a carbocation (Due southN1 pathway) or 2) to be displaced by an incoming nucleophile (which would be an SDue north2 reaction).
Withal we've also seen that treating an alcohol with acid leads to an interesting "personality adjustment": the alcohol (R-OH) is converted to its conjugate acrid, (R-OH2+) which now possesses a decent leaving group (the weak base nosotros know every bit h2o, H2O). We saw, in a previous post, how this allows for formation of (symmetrical) ethers from alcohols, either via SN2 pathway (with principal alcohols) or an SNorthi pathway (tertiary alcohols).
We might enquire: can this exist extended to grade other functional groups as well ethers?
Sure thing!Treating alcohols with HCl, HBr, or HI (which all fall under the grab-all term "HX" where 10 is a halide) results in the germination of alkyl halides.
This occurs in a ii footstep process: first, the alcohol is protonated to give its cohabit acid. Secondly, a substitution occurs.
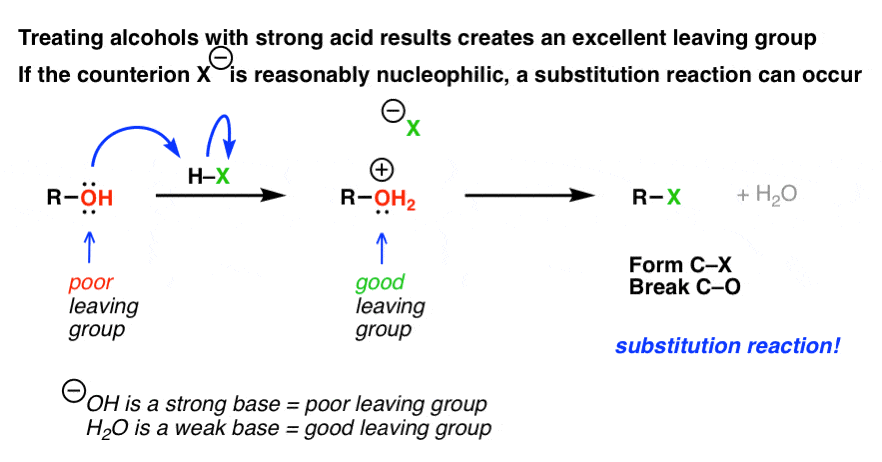
Notice how that second step (substitution) was left vague in the diagram above. That's because, as we've seen, the type of substitution pathway depends on the substrate.
2. Alkyl Halides From Methyl And Chief Alcohols Via SouthN2 Reaction
Knowing how sensitive the SDue north2 reaction is to steric hindrance, nosotros should expect that for methyl booze and for primary alcohols, the SNorth2 pathway dominates. And it does!
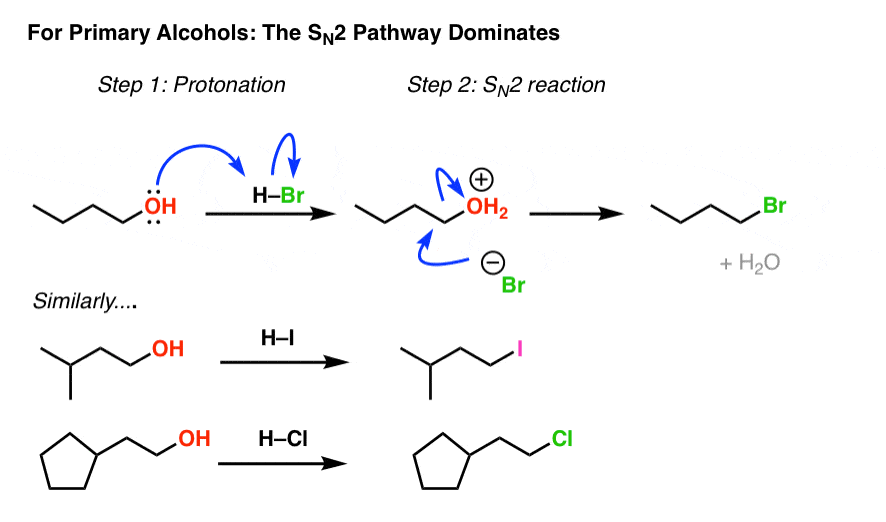
Annotation how in each example we brainstorm by protonating the alcohol, creating a proficient leaving group which is so displaced past the cohabit base of operations of the acid. Alkyl chlorides, bromides, and iodides can each be made this style.
three. Alkyl Halides From Tertiary Alcohols Proceeds Through The SNorthward1 Pathway
Likewise, understanding the trends of carbocation stability, nosotros should expect that conversion of tertiary alcohols to alkyl halides proceeds through an SN1 pathway. And it does.
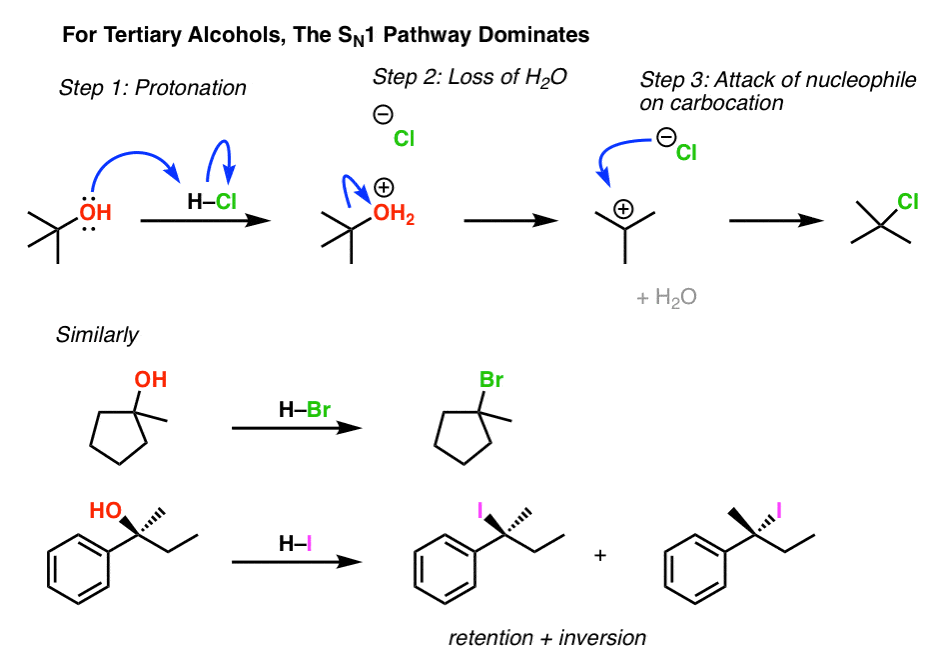
Note in the final example that beginning with a chiral starting material will lead to a mixture of inversion and retention (often called, "racemization") because it goes through the (flat) intermediate carbocation.
iv. A Good Rule of Pollex For Secondary Alcohols With HX: Presume SNorthward1
Methyl, primary, and third alcohols all stand for pretty straightforward cases.
"Then what virtually secondary alcohols?" yous might ask. Ah aye. This is where things go interesting – and is, therefore, the stuff of which exam questions are made.
In the lab, handling of secondary alcohols with HX leads to a mixture of products from SNorthane and SDue north2 pathways. For practical purposes it is generally not a useful procedure, especially if you intendance about preserving stereochemistry.
However, your introductory textbook and course notes are non "the lab". The purpose of a course is to introduce you lot to important concepts in organic chemistry. And from an teacher'south standpoint, it so happens that the conversion of secondary alcohols to secondary alkyl halides by HX is an excellent opportunity to bring up the subject of carbocation rearrangements. This falls nether the purview of the SouthwardN1 pathway. So a good dominion of thumb is to assume – for the purposes of your form – that secondary alcohols treated with HX will proceed through an SNi mechanism.
5. Rearrangements In The Formation Of Alkyl Halides From Alcohols
We've covered rearrangements (hydride and alkyl shifts) earlier in the context of SouthN1 reactions. But it's worth touching on again.
The basic premise is this. Carbocations are unstable, electron-poor species. Their stability generally increases with the number of attached carbons, which serve to donate electron density. Hence, the stability of carbocations increases in the management methyl < primary < secondary < tertiary. Nosotros too saw that carbocations are stabilized by resonance.
As nosotros saw in a previous series of posts – consult this if you demand more than hand holding! – carbocations can undergo ane,2 shifts of C-H and C-C bonds, resulting in new carbocations. Such rearrangements are most likely to occur if they can effect in a more stable carbocation. For case, the rearrangement of a secondary to a 3rd carbocation, is a favoured (energetically "downhill") process, whereas a rearrangement from a 3rd to a secondary carbocation (energetically "uphill") is unlikely.
Anytime a reaction proceeds through a carbocation intermediate, we need to be on the lookout to see if it isnext to a carbon which can generate a more stable carbocation through a shift of a C-H or C-C bond.
In that location are three cases in item to watch out for.
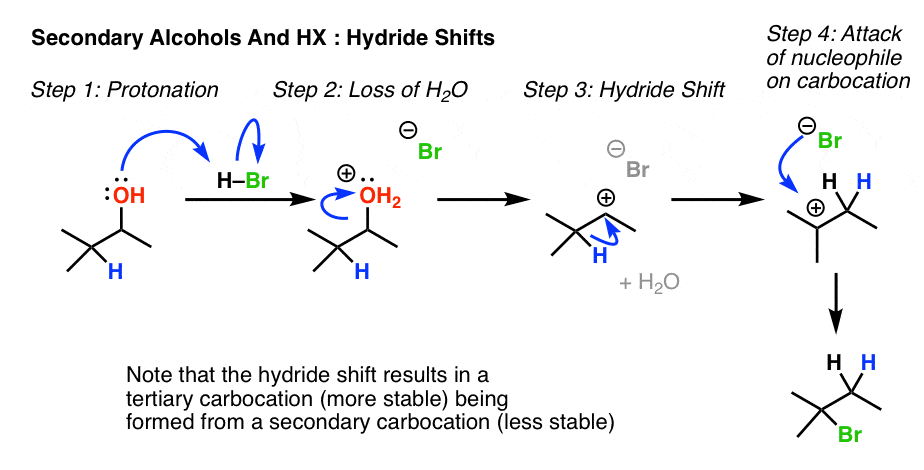
6. Rearrangement Example #1: A Hydride shift
Wait for a secondary alcohol adjacent to a tertiary carbon. Annotation this common case, where protonation leads to loss of water, followed past a hydride shift so trapping of the carbocation by the halide ion.
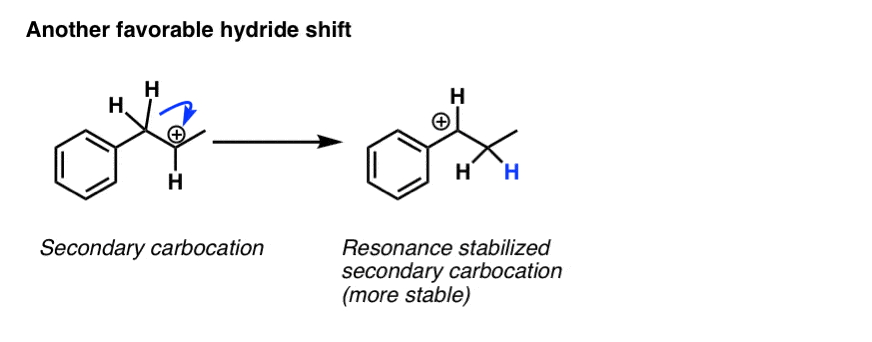
Another instance of a favourable rearrangement is when a secondary carbocation is adjacent to an "allylic" or "benzylic" hydrogen. Rearrangement results in a secondary carbon which is stabilized by resonance.
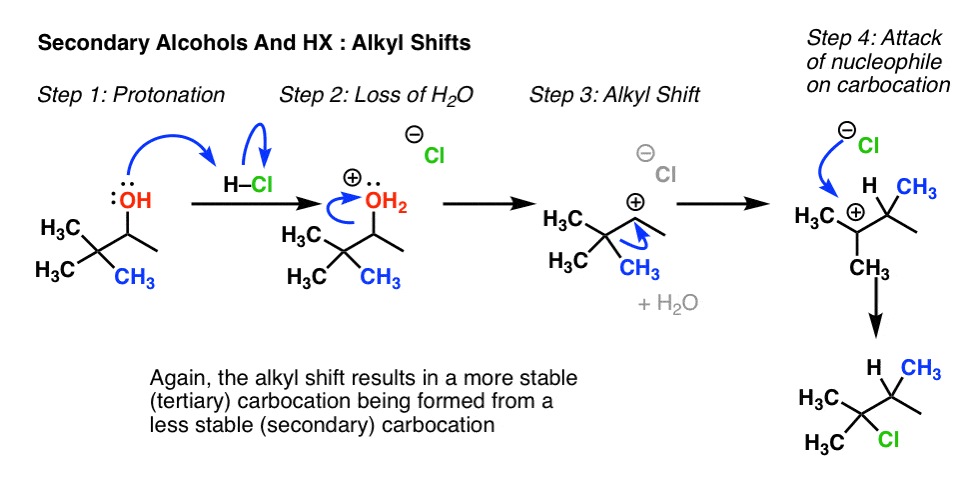
7. Rearrangement Example #2: Alkyl Shift
Wait for a secondary alcohol adjacent to a quaternary carbon (i.e. a carbon attached to 4 other carbons). Annotation how this is essentially the verbal same process as the hydride shift above, except that CH3 is migrating, not H.
viii. Rearrangement Example #3: A Special Instance of Alkyl Shifts – Band Expansions And Contractions
Look for a secondary alcohol that is adjacent to a strained ring (cyclobutane in the classic case). Once the secondary carbocation is generated, a bond in the strained band migrates, leading to expansion of the ring past 1. This is particularly favourable in the example of cyclobutane to cyclopentane since cyclobutane is highly strained (ca 26kcal/mol) whereas cyclopentane has only balmy ring strain.
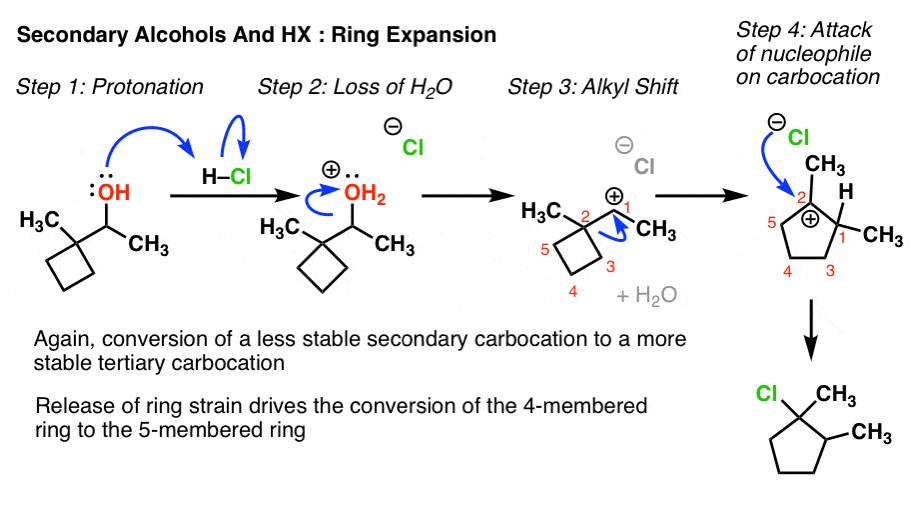
This blazon of alkyl shift normally gives students a hard time, which of course makes it a favourite exam problem of instructors. Although the curved arrow drawn is no unlike than that for the previous two cases, I think the primary difficulty is in mapping the production from the starting material. In this respect I recommend ii things:
- Number the carbons (not necessarily IUPAC – just number to go on rail of them). For instance here the arrow in Step iii (the alkyl shift) shows united states of america breaking C2-C3 and forming C1-C3. Applying the rules of curved arrows implies that C1 will then exist neutral and C2 will become a carbocation. That'southward all that's happening.No other bonds are formed or broken in this step. It takes practice to go this correct.
- Describe the ugly version first. THEN redraw to brand information technology look skilful.
Band contractions are also possible, although are not every bit favourable as the opening of strained rings. The same principles apply.
9. Alkyl Halides From Alcohols: What Doesn't Work?
It's always helpful to know what doesn't piece of work in the formation of alkyl halides from alcohols.
First of all, since we're dealing with substitution reactions here, some familiar rules use.Merely alkyl alcohols (alcohols on spiii hybridized carbons) will undergo SN1 and SN2 reactions. Both the SN1 and SN2 pathways involve buildup of positive charge on carbon, and sp2 and sp hybridized carbocations are extremely unstable. This attempted SN1 of phenol, for example, will fail miserably:
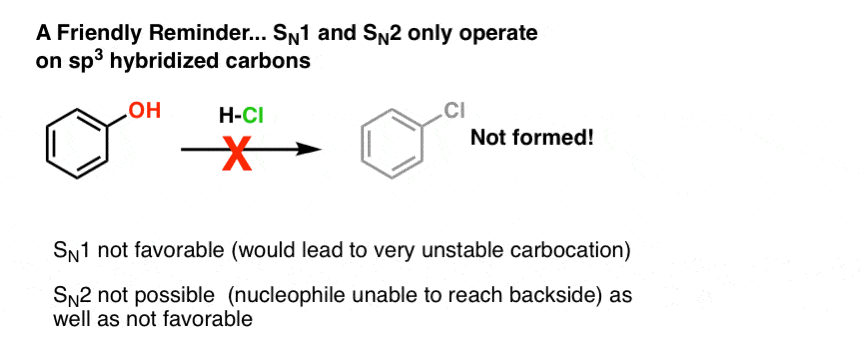
You might as well wonder if we tin apply reagents like HCN, HOAc, or HNthree to convert alcohols to nitriles, esters, and azides respectively. Generally, no. The problem is that each of these are adequately weak acids (pKa four and above) so these will merely requite a low concentration of the protonated alcohol. Since the reaction charge per unit is proportional to concentration, formation of these products will be slow. [With azides, there are as well potential complications with a unlike type of rearrangement, simply as a curtiusy we're not going to bargain with that schmidt right now : – ) ]
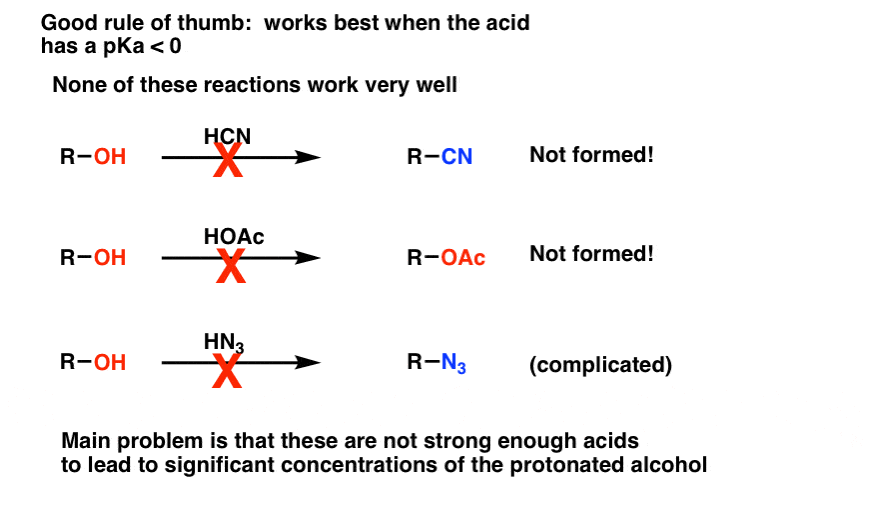
For our purposes, conversion of alcohols to other substitution products using strong acid is limited to HCl, HBr, Howdy, and the special instance of H+/ROH which gives symmetrical ethers. A adept dominion of thumb is that the conjugate acrid of the nucleophile should take a pKa of 0 or less in guild for the reaction to occur.
ten. Summary: Making Alkyl Halides From Alcohols
Then alcohols can be converted to alkyl halides. You might ask, "why should we care?". The reply is that, equally we said, converting an booze (which has a poor leaving grouping) into an alkyl halide (which has a nifty leaving group) at present allows us to do all kinds of functional group interconversions that were not previously possible. The SN2 is a very useful and powerful reaction, for instance. Once a primary alcohol has been converted to a primary alkyl halide, nosotros tin then treat it with all varieties of nucleophiles to make a multitude of functional groups.
Yet squeamish it is to be able to practice this, though, it's far from ideal. Nosotros have to use strong acid, which tin can frequently cause complications if we have acid-sensitive functional groups on our molecule. Furthermore, all those pesky rearrangements on secondary carbons are a hassle. They can screw with our stereochemistry and pb to undesired products. Yous might ask, "isn't in that location some way to get effectually that?".
Yes! We'll talk about a very squeamish way around this dilemma in the adjacent postal service!
Next Post – Tosylates And Mesylates
(Advanced) References and Farther Reading
Alkyl chlorides from alcohols:
- -BUTYL CHLORIDE
James F. Norris and Alanson W. Olmsted
Organic Syntheses, Coll. Vol. 1, p.144 (1941); Vol. 8, p.fifty (1928).
DOI: 10.15227/orgsyn.008.0050
An example of an SouthwardN1 conversion of tert-butanol to t-butyl chloride with HCl. This is from Organic Syntheses, a source of reliable and independently tested organic chemistry experimental procedures.Alkyl iodides from alcohols: - Reaction betwixt unsaturated alcohols and potassium iodide in the presence of polyphosphoric acrid
Richard Jones and J. B. Pattison
J. Chem. Soc. 1969, 1046
DOI: x.1039/J39690001046
This newspaper uses KI + phosphoric acid to generate HI in situ, which converts alcohols to iodides. - i,half-dozen-DIIODOHEXANE
Herman Stone and Harold Shechter
Synth. 1951, 31, 31
DOI: 10.15227/orgsyn.031.0031
A procedure from Organic Syntheses, a source of reliable and independently tested constructed organic experimental procedures, for converting alcohols to iodides with KI + PPA (polyphosphoric acid). - Synthetic methods and reactions. 63. Pyridinium poly(hydrogen fluoride) (30% pyridine-70% hydrogen fluoride): a convenient reagent for organic fluorination reactions
George A. Olah, John T. Welch, Yashwant D. Vankar, Mosatomo Nojima, Istvan Kerekes, and Judith A. Olah
The Journal of Organic Chemical science 1979, 44 (22), 3872-3881
DOI: 1021/jo01336a027
Pyridinium poly(hydrogen fluoride), too known every bit PPHF or "Olah's reagent" can be used as a Bronsted acid for converting alcohols to iodides, forth with KI or NaI. - A Simple, Efficient, and General Method for the Conversion of Alcohols into Alkyl Iodides by a CeCl37H2O/NaI Organisation in Acetonitrile
Milena Di Deo, Enrico Marcantoni, Elisabetta Torregiani, Giuseppe Bartoli, Maria Cristina Bellucci, Marcella Bosco, and Letizia Sambri
The Journal of Organic Chemistry 2000, 65 (9), 2830-2833
DOI: 10.1021/jo991894c
Lewis acids can exist used for this reaction instead of a Bronsted acid, assuasive for milder reaction weather condition. - Directly conversion of alcohols into the respective iodides
Reni Joseph, Pradeep Due south. Pallan, A. Sudalai, T. Ravindranathan
Tetrahedron Lett. 1995 36 (iv), 609-612
DOI: 10.1016/0040-4039(94)02315-3
Elemental iodine (I2) can as well exist used for direct converting alcohols to iodides.Alcohols can be converted to alkyl bromides with PBr3: - Convenient synthesis of labile optically active secondary alkyl bromides from chiral alcohols
Robert O. Hutchins, Divakar. Masilamani, and Cynthia A. Maryanoff
The Journal of Organic Chemistry 1976, 41 (6), 1071-1073
DOI: 1021/jo00868a034 - Synthesis of Optically Active Alkyl Halides
Harry R. Hudson
Synthesis 1969, 112-119
DOI: 1055/s-1969-34195
The principal utility of PBr3 is that it allows the conversion of chiral alcohols to bromides with inversion of configuration without rearrangement, every bit the to a higher place ii papers demonstrate. They as well illustrate the machinery of the reaction, going through the intermediate alkyl phosphites. - TETRAHYDROFURFURYL BROMIDE
L. H. Smith
Org. Synth. 1943, 23, 88
DOI: x.15227/orgsyn.023.0088
This process from Organic Synthesis, a source of reliable and independently tested experimental organic chemistry procedures, shows how PBriii is compatible with ethers.
wunderlichwourethe.blogspot.com
Source: https://www.masterorganicchemistry.com/2015/02/27/making-alkyl-halides-from-alcohols/
0 Response to "what reagents convert an alcohol to alkyl chloride"
Post a Comment